OUR LABORATORY IS EQUIPPED WITH VARIOUS EQUIPMENT AND CAN MEET VARIOUS REQUIREMENTS OF CHEMICAL ANALYSIS AND METALLOGRAPHIC ANALYSIS.
FOR THE SELECTED INSTRUMENT, WE ILLUSTRATE THE FEATURES IN DETAIL, THE OPERABLE FUNCTIONALITY AND ANALYSIS, IN COLLABORATION WITH Tecnologie Superficiali Srl .
POLAROGRAPHY
What is polarography and what is it used for?
Polarography is an electrochemical method for the identification and determination of traces of substances in solution.
Our chemical laboratory is equipped with a polarografer from Amel Instruments.
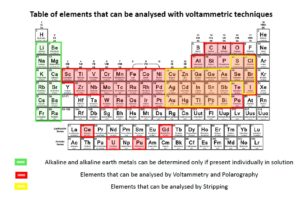
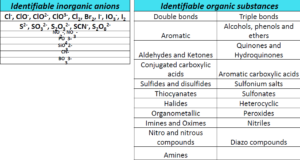
What can we analyse and identify at Metalcoating?
- quantitative or qualitative determination of chemical species in solution in the order of the ppb (micrograms / L). These species must be able to shrink or oxidise
- analysis and identification of traces of heavy metals and organic substances
- speciation of the various ionic forms of the metals in various matrices such as water, food, soil, air, industrial products, pharmaceuticals, cosmetics, biological, etc.
- particularly sensitive (ppb = g / L) for identification and analysis of lead (pb), Cadmium (Cd), copper (Cu), bismuth (Bi), zinc (Zn) and tellurium (Te).
Substances which can be analysed through voltammetric techniques
The instrument is based on the presence of a cell containing the solution to be analysed, constituted by:
- reference electrode, constituted by a large surface area of mercury
- of the outgoing mercury drop that goes on to constitute the second electrode (electrode to drop).
The mercury drop comes out at the rate of one drop every 3-6 seconds, flowing from a hose connected to the container, to a continuous voltage generator, to a potentiometer whose cursor is taken from the voltage to be applied to the cell, and a galvanometer.
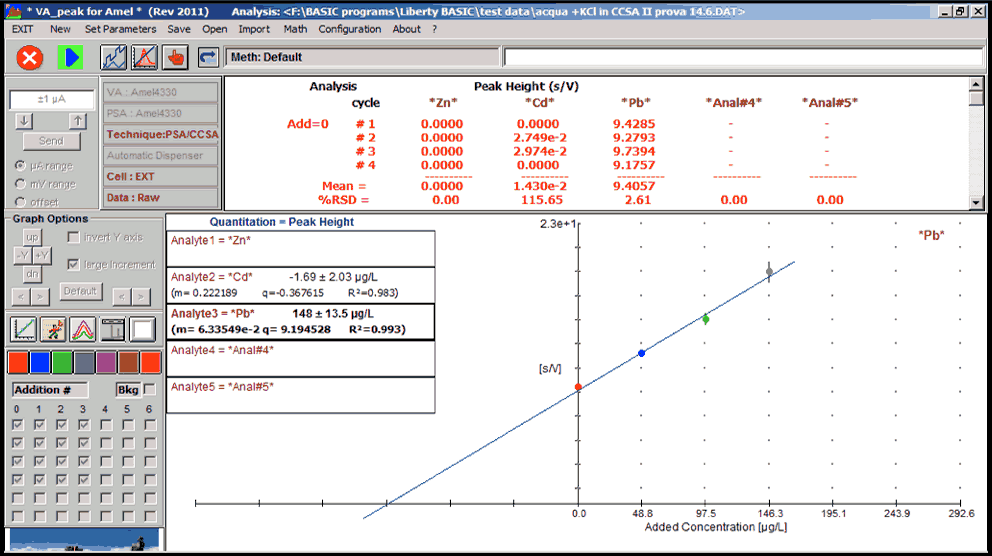
The values of the output current from the galvanometer are rendered in graphic form automatically in function of the voltage (polarogramma).
During the polarographic investigation two conditions are necessary:
- The test solution, in addition to the species to be analysed, has salt added to it (supporting electrolyte) in a concentration approximately 100 times greater than that of the species to be assayed: the ions of this salt have the task of transporting the current in the solution (current migration).
- The analysis takes place in absolute silence.
Such conditions involve the consequence that of the three components of the electrolysis current (current migration, convection current and diffusion current), the first two are void.
The polarographic current is therefore only a diffusion current: this means that it is due to the reaction of the electrode ions joined to it because it is operating a concentration gradient between the body of the solution, which is richer, and the electrode area, which is poorer, than the species that reacts to the electrode. Even if the applied voltage is increased, it cannot grow beyond a certain value (diffusion limit current): from this it follows that the polarogramma is a typical curve obtained in steps, in correspondence to that which the current undergoes an abrupt increase to a small voltage variation. Each step (polarographic wave) corresponds to the reaction of one of the ionic species present in solution; the voltage corresponding to its middle point has the potential of a half-wave, the characteristic of each present ionic species, so as to permit identification (qualitative analysis).
The value reached by the diffusion limiting current is proportional to the concentration of the species that reacts to the electrode; this fact is based on the application of the quantitative analysis.
The sensitivity of the instrument is therefore very high in the order of µg/L.